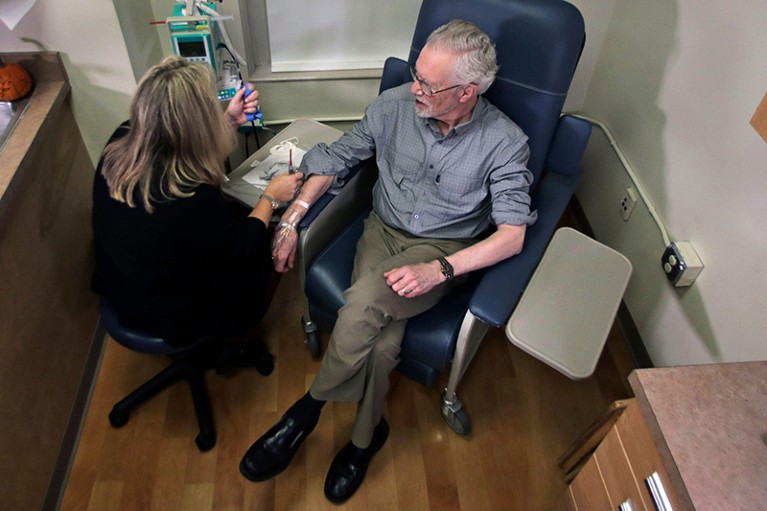
A clinical-trial participant receives the experimental drug aducanumab. White persons are over-represented in trials for Alzheimer’s therapies.Credit score: Charles Krupa/AP Picture
Black and Hispanic persons are as much as twice as doubtless as white individuals to develop Alzheimer’s illness, however they’ve a a lot decrease likelihood of being included in medical trials for Alzheimer’s therapies.
Folks of color made up solely 20% of individuals in trials1 for the Alzheimer’s drug lecanemab, authorized in July 2023, and fewer than 10% within the trial2 for donanemab. The 1,736-person donanemab trial — which was introduced by the pharmaceutical firm Eli Lilly, based mostly in Indianapolis, Indiana, eventually month’s Alzheimer’s Affiliation Worldwide Convention (AAIC) in Amsterdam — included solely 19 Black individuals who acquired the drug.
Conquering Alzheimer’s: a have a look at the therapies of the longer term
The low numbers are making some researchers fear about whether or not these medicine — the primary to point out enhancements to medical outcomes for individuals with Alzheimer’s — will work for individuals of color, and whether or not these trials totally deal with the causes of dementia, which could differ throughout demographics.
“I don’t suppose it must be acceptable that medical trials are so non-representative,” says neurologist Gil Rabinovici on the College of California, San Francisco. “This can be a name to arms.”
Strict necessities
In April 2022, the US Meals and Drug Administration, based mostly in Silver Spring, Maryland, instituted tips recommending that trials mirror the range of people that will use the drug — however this doesn’t at all times occur. The dearth of range is especially acute for Alzheimer’s illness. It’s because individuals in trials testing monoclonal antibody medicine equivalent to lecanemab and donanemab will need to have ample ranges of the sticky amyloid protein that accumulates within the brains of individuals with Alzheimer’s.
On the AAIC, neurologist Doris Molina-Henry, on the College of Southern California in Los Angeles, introduced a examine which discovered that having low amyloid ranges made individuals of color two to 4 instances much less doubtless than their white counterparts to qualify for an ongoing trial testing whether or not lecanemab may forestall Alzheimer’s. A examine3 introduced on the 2022 AAIC assembly discovered related tendencies in knowledge from almost 11,000 individuals within the early levels of Alzheimer’s who underwent positron emission tomography (PET) scans to find out whether or not they may take part in 4 separate Alzheimer’s trials run by Eisai, a bio-pharmaceutical firm based mostly in Tokyo.
May medicine forestall Alzheimer’s? These trials intention to search out out
Qualifying for the most recent donanemab trial was much more troublesome, says Lilly’s senior medical director, John Sims, as a result of the corporate was screening for each amyloid and tau, one other Alzheimer’s-related protein. Just one in 8 Black and one in 17 Hispanic volunteers certified, he says, in contrast with one in 4 white candidates.
“The sector actually wants to know why this retains taking place,” says Alzheimer’s researcher Joshua Grill on the College of California, Irvine. It’s unclear why individuals of color would have decrease amyloid or tau ranges than their white counterparts with the identical quantity of cognitive impairment. Grill speculates that dementia in individuals of color would possibly usually be attributable to different circumstances equivalent to vascular issues or irritation. Exterior components equivalent to training ranges and stress may additionally contribute to dementia threat, and a few proof means that sure genetic variants concerned in Alzheimer’s threat differ between individuals of European and African ancestry4,5.
Amyloid ranges should not the one purpose that Alzheimer’s trials lack racial range, says Reisa Sperling, a neurologist at Harvard College in Cambridge, Massachusetts. Folks of color are much less doubtless than white individuals to reside close to hospitals with PET scanners which are used to find out whether or not a drug is working, and recruitment campaigns usually goal white communities. Folks of color additionally face greater charges of issues that disqualify them from trials, equivalent to heart problems and lupus. “We have to have a look at all our inclusion and exclusion standards: that are vital and that are contributing to disparities in who we usher in,” Sperling says.
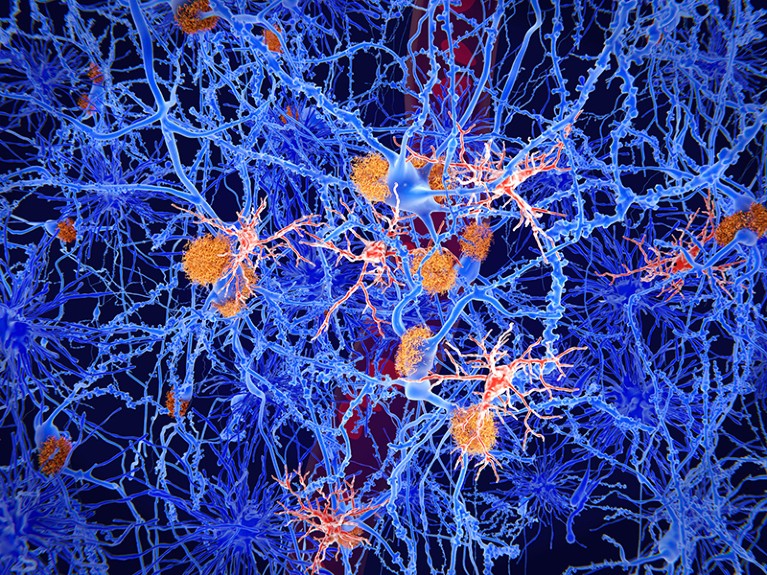
An illustration of amyloid plaques (orange) in mind tissue.Credit score: Getty
Grill and others are involved about whether or not lecanemab and donanemab shall be secure and efficient in various populations. Current trials have proven that monoclonal antibodies can gradual cognitive decline by round 30% in chosen teams of individuals with Alzheimer’s who’ve gentle cognitive impairment. However neither drug stops the development of the illness, and each continuously trigger mind abnormalities that may result in haemorrhages, seizures or dying. Sims says that Lilly’s newest donanemab trial had too few individuals who had been individuals of color to find out whether or not these dangers, and even the drug’s effectiveness, differ by race.
Heralded Alzheimer’s drug works — however security issues loom
That issues Jennifer Manly, a neuropsychologist at Columbia College in New York Metropolis, who says that she would hesitate to suggest monoclonal antibodies if a Black member of the family had Alzheimer’s. “I’d wish to know individuals within the medical trials had been as near my members of the family as they’ll get — their lived expertise, background and well being dangers,” she says. “We don’t have that for Black and Hispanic individuals proper now.”
The dearth of range in medical trials will not be solely an fairness drawback but in addition a scientific one, Manly says, as a result of it may forestall researchers from figuring out the causes of Alzheimer’s and different types of dementia. The restricted success of monoclonal antibodies in opposition to amyloid has made it more and more clear that Alzheimer’s is not only pushed by amyloid or tau, she says. Focusing medicine solely on these proteins would possibly miss different components that contribute to the illness throughout populations. “It’s not solely about creating the therapy however understanding the variations to allow them to inform the design of future research,” says Molina-Henry.
Wider participation
Eisai is now working with neighborhood teams equivalent to church buildings to promote its trials extra broadly, says Shobha Dhadda, Eisai’s senior vice-president of biostatistics and medical improvement operations for neurology. Step one of its Alzheimer’s prevention trial will display potential individuals for amyloid ranges with a brand new blood-based take a look at, which ought to assist to rule out individuals who don’t qualify with out forcing them to undergo cognitive exams or PET scans. Lilly can also be ramping up related recruitment methods in its donanemab trials: Sims says that it has doubled the variety of individuals of color taking part in a 1,000-person security trial, which can conclude later this yr. It’s also switching from PET scans to blood exams in an ongoing trial testing whether or not donanemab can forestall Alzheimer’s.
Grill would additionally wish to see extra infrastructure for Alzheimer’s registries that may direct disqualified people to different trials that might assist them extra. “I’ve nice concern that by turning them away they’ll return to their neighborhood and say, ‘They didn’t need me,’” he says. “We don’t wish to add to the stigma of illness or decrease belief locally.”